Shapes of molecules:
VSEPR THEORY
VALENCE SHELL ELECTRON REPULSION THEORY.
shape of a molecule depends only on central atom and the lone pairs on central atom.
Case#》No# of lone pairs 》Bond angle 》Shape
AB2》 0 》180º 》 Linear
AB2》 1 》about 》120º 》Bent/ V shape
AB2 》2 》104.5º 》 Bent/ V shape
AB3 》0 》exactly 》120º 》Trigonal planner
AB3 》1 》107.5º 》Trigonal / Pyramidal
AB4 》0 》109.5º 》Tetrahedral
AB5 》0 》90º, 120º》 Bipyramidal
AB6 》0 》90º 》Octahedral
Shapes drawn:
Linear:
CO2
O=C=O
B—-A—-B
Bent/V shape:
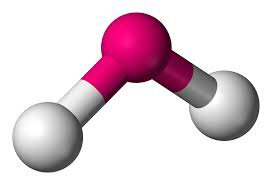
Trigonal planner:
B
B—-A—-B

Trigonal / Pyramidal:
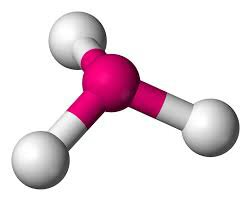

Tetrahedral:

Bipyramidal:


Octahedral:
